electron configuration manganese|complete electron configuration for manganese : Tagatay Scientist Niels Bohr was the first to give an idea of the atom’s orbit. He provided a model of the atom in 1913. The complete idea of the . Tingnan ang higit pa Centris, Edsa. Units D,E,F,G Cyber Pod Two, ETON Centris, Edsa corner Quezon Avenue, QC. For appointment, please call: 0919-082-8774 or email
[email protected]. LABORATORY.
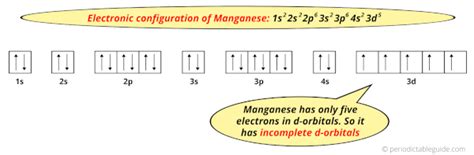
electron configuration manganese,Atoms can jump from one orbital to another orbital in an excited state. This is called quantum jump. The ground-state electron configuration of manganese is 1s2 2s2 2p6 3s2 3p6 3d5 4s2. In the manganese ground-state electron configuration, the five electrons of the 3d orbital are located in the dxy, dyz, . Tingnan ang higit pa

The total number of electrons in manganeseis twenty-five. These electrons are arranged according to specific rules in different orbitals. The arrangement of electrons . Tingnan ang higit paScientist Niels Bohr was the first to give an idea of the atom’s orbit. He provided a model of the atom in 1913. The complete idea of the . Tingnan ang higit pa
The electron configuration of manganese shows that the last shell of manganese has two electrons and the d-orbital has a total of five electrons. Therefore, the valence electrons of manganeseare seven. . Tingnan ang higit pa

Atomic energy shells are subdivided into sub-energy levels. These sub-energy levels are also called orbital. The most probable . Tingnan ang higit pa
Mar 23, 2023
The atomic structure of Manganese includes four electron subshells. First subshell contains 2 electrons; Second subshell contains 8 electrons; Third subshell .
To write the configuration for the Manganese ions, first we need to write the electron configuration for just Manganese (Mn). We first need to find the number of electrons for the Mn atom.
Electron configuration The arrangements of electrons above the last (closed shell) noble gas. Melting point The temperature at which the solid–liquid phase change occurs. .Electron configuration 3d 5 4s 2: Electrons per shell: 2, 8, 13, 2: Physical properties; Phase at STP: solid: Melting point: 1519 K (1246 °C, 2275 °F) Boiling point: 2334 K (2061 °C, 3742 °F) Density (near r.t.) 7.21 g/cm 3: .Electron configuration of manganese. The full electron configuration of this element is 1s2 2s2 2p6 3s2 3p6 3d5 4s2, while its shortened version of electron configuration is [Ar] . The electron configuration of the Manganese element basically states the procedure of distributing the electrons to the atomic orbitals. This whole process or the equation of the electron distribution is . Electron configuration of Manganese is [Ar] 3d5 4s2. Possible oxidation states are +2,3,4,7. Electron Configuration. The periodic table is a tabular display of the .Melting point: 1244 ℃. Density: 7.44 g/cm 3 . Electronic configuration of the Manganese atom in ascending order of orbital energies: 1s 2 2s 2 2p 6 3s 2 3p 6 4s 2 3d 5Electronic .electron configuration manganese complete electron configuration for manganeseAssertion :Manganese (atomic number 25) has a less favourable electron affinity than its neighbours on either side. Reason: The manganese has stable, [ A r ] 18 3 d 5 4 s 2 electrons configuration. View SolutionDans le tableau périodique, les éléments sont classés par ordre croissant de numéro atomique Z. La configuration électronique du manganèse est [Ar] 3d5 4s2. Les états d’oxydation possibles sont +2,3,4,7. Les états d’oxydation les plus courants du manganèse sont +2, +3, +4, +6 et +7, bien que tous les états d’oxydation de -3 à . Explanation: Mn has an atomic number of 25. Simply use this information to obtain its electronic configuration. Mn: 1s22s22p63s23p64s23d5 or simply [Ar]4s23d5 . Answer link. [Ar] 4s^2 3d^5 Mn has an atomic number of 25. Simply use this information to obtain its electronic configuration. Mn: 1s^2 2s^2 2p^6 3s^2 3p^6 4s^2 3d^5 or simply . Subsequently, the s orbital will contain 2 electrons, the p orbital will contain 2 electrons and then the s orbital will have the 10 electrons at max. At last, we will have the following equation of the electrons configuration for Manganese as 1s22s22p63s23p64s23d5. So, this is how we will get our final equation for the Mn .Electron configuration of Manganese is [Ar] 3d5 4s2. Possible oxidation states are +2,3,4,7. The most common oxidation states of manganese are +2, +3, +4, +6, and +7, though all oxidation states from −3 to +7 have been observed. Mn2+ often competes with Mg2+ in biological systems.The electron configuration and the orbital diagram are: Following hydrogen is the noble gas helium, which has an atomic number of 2. The helium atom contains two protons and two electrons. The first electron has the same four quantum numbers as the hydrogen atom electron ( n = 1, l = 0, ml = 0, ms = +1 2 m s = + 1 2 ). What is the electron configuration of manganese? 5. What oxidation states can manganese take? Answers. 1. Mn (s) + F 2 (g) → MnF 2 (s) 2. Manganese is not reactive with water under normal circumstances. 3. Manganese is known for its use in the steel industry. It is used to enhance the properties of steel to make alloys that are .
electron configuration manganeseLa configuration électronique du manganèse est 1s2 2s2 2p6 3s2 3p6 3d5 4s2. Le manganèse est l'élément chimique du tableau périodique des éléments situé dans le groupe 7, son symbole est Mn et son numéro atomique est 25. On le trouve dans la nature sous forme d'élément libre, souvent combiné avec du fer et d'autres minéraux.complete electron configuration for manganese Manganese, on the other hand, has an electron configuration of 1s 2 2s 2 2p 6 3s 2 3p 6 4s 2 3d 5 and a noble gas configuration of [Ar]4s 2 3d 5, resulting in one unpaired electron in each 3d sub-orbital. Similar to copper, the exchange energy is maximized since the 3d sub-orbital is exactly half-filled with 5 electrons without the need . The electron configuration of manganese, atomic number 25, is 1s2222p63s23p63d54s2. The diagram below represents the electron configuration as an orbital diagram. Answer link. Refer to the explanation. The electron configuration of manganese, atomic number 25, is "1s"^2"2"^2"2p"^6"3s"^2"3p"^6"3d"^5"4s"^2".
Electron atomic and molecular orbitals A Bohr diagram of lithium. In atomic physics and quantum chemistry, the electron configuration is the distribution of electrons of an atom or molecule (or other physical structure) in atomic or molecular orbitals. For example, the electron configuration of the neon atom is 1s 2 2s 2 2p 6, meaning that the 1s, 2s, and .
To write the orbital diagram for the Manganese (Mn) first we need to write the electron configuration for just Mn. To do that we need to find the number of .The electronic configuration of Manganese will be 1s2 2s2 2p6 3s2 3p6 3d5 4s2. How do you write the electron configuration for Manganese? The electronic configuration of Manganese will be 1s2 2s2 2p6 3s2 .Manganese (Mn) has an atomic mass of 25. Find out about its chemical and physical properties, states, energy, electrons, oxidation and more. ChemicalAid. . Electron Configuration [Ar] 4s 2 3d 5: 1s 2 2s 2 2p 6 3s 2 3p 6 4s 2 3d 5: Orbital Diagram. Nuclear. Radioactive: No: Isotopes. Symbol Mass Number Relative Atomic Mass Isotopic . In the case of first row transition metals, the electron configuration would simply be [Ar] 4s x 3d x. The energy level, "n", can be determined based on the periodic table, simply by looking at the row number in which the element is in. However, there is an exception for the d-block and f-block, in which the energy level, "n" for the d block is .Electron Configurations are an organized means of documenting the placement of electrons based upon the energy levels and orbitals groupings of the periodic table. The electron configuration for the first 10 elements. H .
Example of Determining Energy Levels (n) For example, if we want to determine the electron configuration for Cobalt (Co) at ground state, we would first look at the row number, which is 4 according to the periodic table below; meaning n = 4 for the s-orbital.In addition, since we know that the energy level for the d orbital is "n-1", therefore .
マンガンの電子配置は1s2s2p2 2s6 3p2d3s6です。. マンガンは、グループ3にある元素の周期表の化学元素であり、その記号はMnで、原子番号は5です。. 自然界では遊離元素として見られ、鉄や他のミネラルと組み合わされることがよくあります。. マンガンは遊離 .
electron configuration manganese|complete electron configuration for manganese
PH0 · manganese electron configuration ground state
PH1 · manganese electron configuration full
PH2 · how to determine number of valence electrons
PH3 · how many valence electrons does manganese have
PH4 · electron configuration for every element
PH5 · electron configuration chart
PH6 · electron configuration calculator
PH7 · complete electron configuration for manganese
PH8 · Iba pa